Fish Oil for Prevention of Sudden Death in Hemodialysis Patients?
Kidney Int. 2013;83(6):993-995.
Friedman et al. report that hemodialysis patients with the highest levels of n-3 fatty acids had impressively low odds of sudden cardiac death. The study is limited by a small sample size, and the analysis relies on only a single baseline measurement of blood levels. Recent randomized evidence indeed fails to support that n-3 fatty acids may prevent sudden death in nonrenal patients. More evidence is needed to advocate fish oil in this setting.
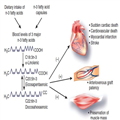
Dialysis patients have dramatically low survival rates, between 35 and 45% at 5 years. Cardiovascular disease is responsible for 43% of deaths in this setting, and sudden cardiac death has now emerged as the leading single cause of mortality, accounting for approximately 25% of deaths. Sudden death is defined as an unexpected 'natural' death within a very short time period in a person without any prior condition that would appear fatal. Data from the general population indicate that about 80% of sudden deaths may be due to ventricular fibrillation. The mechanisms causing the extraordinarily excessive rate of sudden deaths in dialysis patients are largely unknown. A variety of kidney- and dialysis-specific factors may favor the occurrence of this complication, including left ventricular hypertrophy, heart failure, volume overload, myocardial fibrosis, hyperkalemia, hyperphosphatemia, QT dispersion, QT-prolonging medication, sympathetic overactivity/autonomic nerve dysfunction, and electrolyte and volume shifts during hemodialysis (HD) sessions.[1] Because sudden death may derive from many causes, its prevention is a difficult challenge for nephrologists. Successful preventive strategies applied in the general population, such as the use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, statins, and implantable cardioverter-defibrillators, do not appear to offer benefit in dialysis patients.[1]In this pessimistic context, novel routes targeting the risk of sudden death in this population are most welcome.
Long-chain n-3 fatty acids, also called ω-3 fatty acids, are polyunsaturated fatty acids commonly found in marine and plant oils. Their chemical structure includes a double bond (C=C), starting after the third carbon atom from the end of the carbon chain. These fatty acids are essential nutrients for cell-membrane structure and physiological functions such as platelet aggregation and lipid metabolism. A major role for n-3 fatty acids was suggested in the 1970s when extremely low rates of death from cardiovascular disease were reported among the Inuit, as compared with northern European counterparts, despite similar high intakes of fat, about 40% of calories. High amounts of n-3 fatty acids from fish and fish-eating mammals were actually the main sources of fat among the Inuit. In the past decade, in vitro studies, animal experiments, observational studies, and some randomized controlled trials (RCTs) have demonstrated that n-3 fatty acids had beneficial effects on major cardiovascular outcomes, including sudden death, due to antiatherogenic, anti-inflammatory, antithrombotic, antihypertensive, and triglyceride-lowering effects.[2] ω-3 fatty acids have distinct antiarrhythmic effects on myocyte electrophysiology—for example, alteration of the function of membrane sodium channels, L-type calcium channels, and sodium–calcium exchangers.[2] There are three major n-3 fatty acids: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), found in fatty fish (salmon, bluefish, mackerel, arctic char, sardines, and swordfish), and α-linolenic acid (ALA), found in specific vegetable oils (soybean, rapeseed, and flaxseed), walnuts, and some green vegetables (brussels sprouts, kale, spinach, and salad greens). The human organism may partially convert ALA to EPA and DHA, thus allowing vegans to get sufficient amounts of n-3 fatty acids. Western diets typically contain high amounts of saturated fats and insufficient amounts of n-3 fatty acids. Thus, some but not all current guidelines from major societies recommend the use of n-3 fatty acids, either through diets or as supplements.
The question of whether dialysis patients should be supplemented with n-3 fatty acids is appealing. Friedman et al. [3] (this issue) analyzed data from 100 patients who died from sudden cardiac death during their first year of dialysis treatment and 300 frequency-matched control survivors from a US cohort of HD units. Blood levels of long-chain n-3 fatty acids, measured at the time of dialysis initiation, were inversely associated with the risk of sudden cardiac death during the first year of HD, even after adjustment for multiple potential confounders. The odds of sudden cardiac death at 1 year of patients in the two highest quartiles for blood levels of long-chain n-3 fatty acids were remarkably low: 0.22 and 0.20 for the third and fourth upper quartiles, respectively, compared with the lowest quartile. Although these data are encouraging, the study has limitations, including the small size of the patient sample and the fact that the analyses were based on a single baseline measurement that may not reflect levels of n-3 fatty acids over a long period. The initiation of a dialysis program indeed determines a number of changes that may affect blood levels and profiles of n-3 fatty acids, from the baseline value. These modifications include a spontaneous increase in food intake, changes in pharmacological treatment that may interact with blood lipids, and improvement in physical activity. Interestingly, rates of hyperkalemia during the 12 months of follow-up were similar between cases of sudden death and controls. However, other variables that may have contributed to sudden deaths were not measured or recorded during the follow-up—for instance, interdialytic weight gains, electrolyte and volume shifts during HD, and smoking habits. In spite of these limitations, the study demonstrates a strong association between blood levels of n-3 fatty acids and sudden cardiac death in this population. Furthermore, the dramatically low odds of sudden cardiac death in patients from the top quartiles of blood levels of n-3 fatty acids, in the context of biological plausibility, are suggestive of causation.
The story of long-chain n-3 fatty acids is not new to nephrologists. In 1994, Donadio et al.showed that supplementation with fish oil remarkably slowed the progression of IgA nephropathy.[4] After a long period of silence in the field of nephrology, fish oil received renewed attention in 2012, thanks to a Canadian RCT in which fish oil ingestion was found to reduce thrombosis from new HD grafts and to improve cardiovascular-event-free survival in HD patients.[5] It is noteworthy that these end points were secondary outcomes of the study. The primary outcome, the proportion of grafts with loss of native patency, was not statistically improved. Taken together, these recently published studies in HD patients raised new hopes and, according to their authors, should prompt RCTs with fish oil supplementation to improve cardiovascular outcomes in this setting.
A decade ago, enthusiasm for n-3 fatty acids was sky high, with medical evidence from studies in nonrenal patients showing that ω-3s provided by food or supplements had a strong cardiovascular protective effect across all major cardiovascular outcomes.[6]Unfortunately, since then, the picture has clouded. Although epidemiological studies have consistently shown that diets rich in n-3 fatty acids or blood levels of n-3 fatty acids were associated with impressively low odds of sudden death,[7] results from prospective trials in which n-3 fatty acids were supplemented through diets or pills have provided conflicting results. The protective effect shown initially became non-significant, as more randomized evidence accumulated.[8] In 2012, a large meta-analysis including 20 RCTs and almost 70,000 patients failed to show any benefit in the prevention of major cardiovascular end points such as cardiac death, myocardial infarction, and stroke.[9] In the latter meta-analysis, sudden death was examined in seven RCTs, including 41,751 patients and 1030 events, and yet n-3 fatty acids failed to reduce the odds of sudden death, thus rejecting a distinct antiarrhythmic effect. Finally, a double-blind RCT involving 12,536 participants at high risk for cardiovascular events (patients with diabetes, impaired fasting glucose, and impaired glucose tolerance) was equally negative regarding cardiovascular events, including deaths from arrhythmias.[10] The failure to replicate earlier positive findings with n-3 fatty acids may be explained by the analysis of much larger studies, the improvement in the quality of the studies, and the systematic use of state-of-the-art concomitant treatments—antihypertensive, antithrombotic, and statins—to manage cardiovascular disease. Discrepancies between positive epidemiological evidence in favor of fish or ω-3 eaters and the absence of effect in prospective RCTs are inevitably linked to the fact that people eating a lot of fish are more likely to benefit from a healthier diet with less saturated fats from hamburgers and processed foods, than are those consuming little or no fish.
Of course, this compelling negative evidence in nonrenal patients does not exclude the possibility that n-3 fatty acids might be efficient in reducing rates of sudden death in extremely high-risk individuals, such as those initiating dialysis. Dialysis patients may indeed benefit from n-3 fatty acids in various indications (Figure 1). However, the lessons learned from studies in non-dialysis settings, coupled with the consistent history of negative trials in the dialysis population, should invite caution. Further steps may be required before investment of resources in an RCT with n-3 fatty acids in this population. First, additional epidemiological evidence linking sudden death and levels of n-3 fatty acids should be obtained from larger samples of HD patients. Second, relationships between diets and blood levels of n-3 fatty acids, and type of n-3 fatty acids, in HD patients in diverse countries and ethnic groups should be obtained, in order to target people who are more likely to benefit from supplementation. Third, a dose–response curve should be sought, in order for an interventional trial to reach blood levels of n-3 fatty acids matching those from the top quartiles in the study by Friedman et al. [3] Fourth, the safety of n-3 fatty acids should be more precisely addressed if large samples of HD patients are being tested. Although fish oil is generally harmless, it theoretically might increase the risk of bleeding especially in patients taking aspirin, clopidogrel, and anticoagulants, which are prevalent in this population. A last philosophical consideration is that miracle ω-3 pills may be no substitute for a healthier lifestyle including eating fish at least twice a week, getting lots of physical activity, and not smoking.
No comments:
Post a Comment